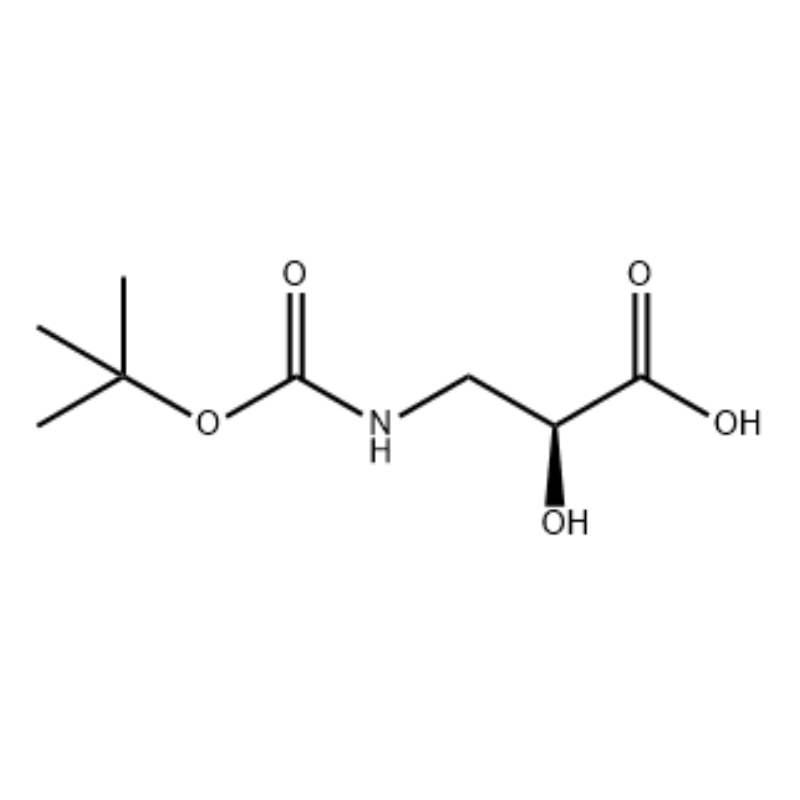
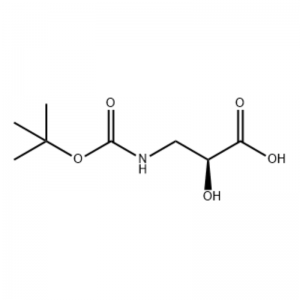
(S) -isoserine 15a (21 g, 0.20 mol) an narkar da shi a cikin tetrahydrofuran (100 mL) Kuma gauraye da sauran ƙarfi na 10% mai ruwa sodium hydroxide bayani (100 ml), Di-tert-butyl dicarbonate (50 ml, 0.22 mol) ya kasance. an kara daɗaɗɗa. An gudanar da aikin a cikin dakin da zafin jiki na tsawon sa'o'i 9. An daidaita yanayin ruwa zuwa pH 2 tare da 4 mol / L hydrochloric acid, kuma an fitar da shi tare da dichloromethane / methanol (v / v = 5/1, 50 mL × 3). ) da kuma bushe a kan anhydrous sodium sulfate.Tace ta tsotsa, mayar da hankali a karkashin rage matsa lamba, The title fili 15b aka samu a matsayin mai launi mara launi (35 g, yawan amfanin ƙasa: 85%).
Zuwa wani bayani mai motsawa na S-isoserine (4.0 g, 0.038 mol) a cikin dioxane: H2O (100 ml, 1: 1 v / v) a 0 ° C an kara N-methylmorpholine (4.77 mL, 0.043 mol), sannan BoC2O ya biyo baya. (11.28 ml, 0.049 mol) kuma an motsa dauki cikin dare tare da ɗumamar hankali zuwa zafin jiki.Glycine (1.0 g, 0.013 mol) an ƙara sannan kuma an motsa shi don 20 min.An kwantar da martani ga O0C kuma ya zauna aq.NaHCO3 (75 ml) an ƙara.An wanke Layer mai ruwa tare da ethyl acetate (2 x 60 ml) sannan kuma an sanya shi acidified zuwa pH 1 tare da NaHSO4.An fitar da wannan maganin tare da ethyl acetate (3 x 70 mL) kuma an bushe waɗannan nau'o'in nau'in kwayoyin halitta a kan Na2SO4, an tace su kuma mayar da hankali ga bushewa don ba da N-Boc-3-ammo-2 (S) -hydroxy-propanoic acid. (6.30 g, 0.031 mmol, kashi 81.5 yawan amfanin ƙasa): 1H NMR (400 MHz, CDC13) δ 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m , 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H);13C NMR (IOO MHz, CDC13) δ 174.72, 158.17, 82, 71.85, 44.28, 28.45.
N-Boc-3-amino-2 (S) -hydroxy-propionic acid;Zuwa wani bayani mai motsawa na S-isoserine (4.0 g, 0.038 mol) a cikin dioxane: H2O (100 ml, 1: 1 v / v) a 0 ° C an kara N-methylmorpholine (4.77 mL, 0.043 mol), sannan BoC2O ya biyo baya. (11.28 ml, 0.049 mol) kuma an motsa dauki cikin dare tare da ɗumamar hankali zuwa zafin jiki.Glycine (1.0 g, 0.013 mol) an ƙara sannan kuma an motsa shi don 20 min.An kwantar da martani zuwa 0°C kuma ya zauna aq.NaHCO3 (75 ml) an ƙara.An wanke Layer mai ruwa tare da ethyl acetate (2 x 60 ml) sannan kuma an sanya shi acidified zuwa pH 1 tare da NaHSO4.An fitar da wannan maganin tare da ethyl acetate (3 x 70 mL) kuma an bushe waɗannan nau'o'in kwayoyin halitta a kan Na2SO4, an tace su kuma mayar da hankali ga bushewa don ba da N-Boc-3-amino-2 (5) -hydroxy-propanoic acid. (6.30 g, 0.031 mmol, kashi 81.5 yawan amfanin ƙasa): 1H NMR (400 MHz, CDC13) δ 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m , 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H);13C NMR (100 MHz, CDC13) δ 174.72, 158.17, 82, 71.85, 44.28, 28.45.
 Ginin 12, No.309, Titin 2 ta Kudu, yankin raya tattalin arziki, gundumar Longquanyi, Chengdu, Sichuan, kasar Sin.
Ginin 12, No.309, Titin 2 ta Kudu, yankin raya tattalin arziki, gundumar Longquanyi, Chengdu, Sichuan, kasar Sin. amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com
amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com +86 (028) 84841969
+86 (028) 84841969 +86 135 5885 5404
+86 135 5885 5404

















.png)


