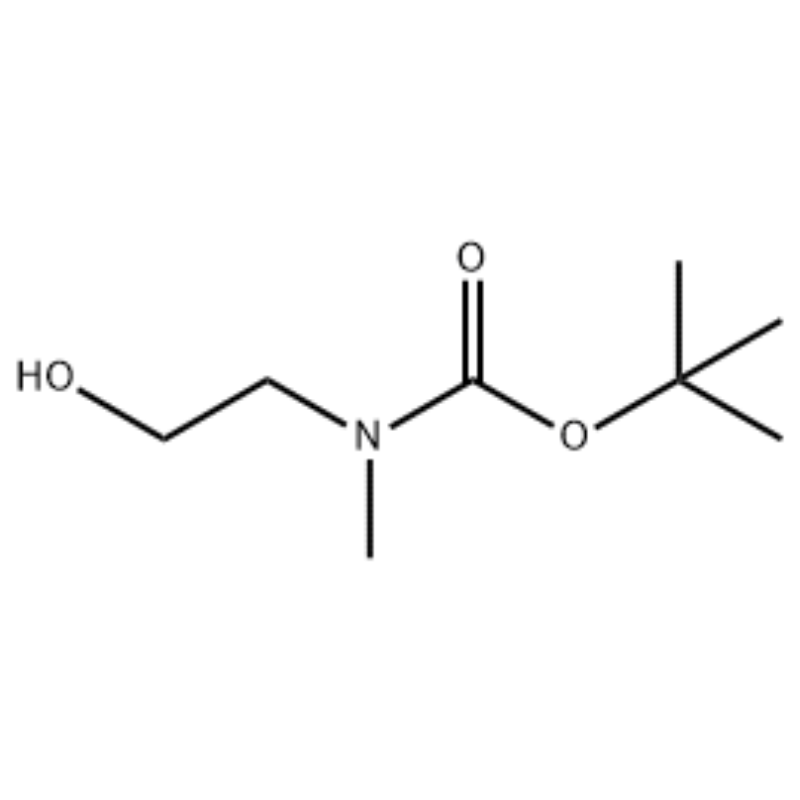
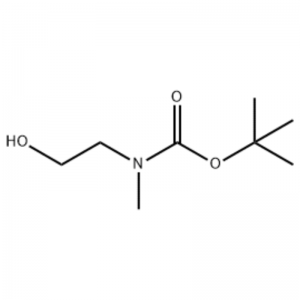
Zuwa wani bayani na 2- (methylamino) ethanol (500 MG, 0.53 ml, 6.66 mmol) a cikin CH2Cl2 (20 ml) an ƙara Boc2O (1.48 g, 6.79 mmol), sannan ya motsa a dakin da zafin jiki na awa 1.An fitar da maganin dauki tare da brine da CH2Cl2.An bushe Layer Layer don haka aka bushe akan MgSO4 kuma an tace shi.Sa'an nan kuma, an mayar da tacewa a cikin vacuo don samun abun da ke ciki (mai marar launi, ƙididdiga);1H NMR (200 MHz, CDCl3) delta 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H);babban bakan m/e (ƙarfin dangi) 144 (20) 102 (24) 57 (70) 44 (100).
Misali 38;N1- (3-Fluoro-4- (2- (1- (2- (methylamino)) ethyl) -1H-imidazol-4-yl) thieno [3,2-b] pyridin-7-yloxy) phenyl) -N3 -(2-methoxyphenyl) malonamide (96);Mataki na 1: tert-Butyl 2-hydroxyethyl (methyl) carbamate (97) (J. Med. Chem., 1999, 42, 11, 2008) Zuwa maganin 2- (methylamino) ethanol (5.0 g, 67 mmol) a ciki THF (50 ml) a RT an ƙara Boc2O (15.7 g, 72 mmol) kuma an zuga cakuda dauki a RT don 4 hours.An mayar da hankali kan cakuda ga bushewa kuma an yi amfani da fili mai suna 97 kai tsaye a mataki na gaba ba tare da ƙarin tsarkakewa ba (11.74 g, 100% yawan amfanin ƙasa).MS (m/z): 176.2 (M+H).
Shiri na l-2-[4-Bromo-2- (4-oxo-2-ftiotaioxo1hiazolidin-5-ylidenemefliyl) phenoxy] efliyl-3-efliyl-l- methylurea (Compoiotamd 161) Mataki na 1: Haɗin t-butyl2- hydroxyethylmethylcarbamate;Zuwa wani bayani na 2- (methylamino) ethanol (500 MG, 0.53 ml, 6.66 mmol) a cikin CH2Cl2 (20 ml) an ƙara BoC2O (1.48 g,6.79 mmol), sa'an nan kuma motsawa a dakin da zafin jiki na 1 hour.An fitar da maganin dauki tare da brine da CH2Cl2.An bushe Layer Layer don haka aka bushe akan MgSO4 kuma an tace shi.Sa'an nan, filtrate aka mayar da hankali a cikin vacuo don samun abu fili (mai launi, adadi); 1HNMR (200 MHz, CDCl3) delta 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H);babban bakan m/e (ƙarfin dangi) 144 (20) 102 (24) 57 (70) 44 (100).
2- (methylamino) ethanol (90.1 g, 1.2 mol) an narkar da shi a cikin 1.2 L na methylene chloride, kuma BoC2O (218 g, 1 mol) an ƙara shi a hankali yayin da yake motsawa a 00C, sannan kuma a dakin da zafin jiki na 3 hours.An wanke cakudar da aka yi bi da bi tare da 700 ml na maganin ruwa mai cikakken ammonium chloride, da 300 ml na ruwa.An wanke cakuda da aka wanke ta hanyar amfani da sodium sulfate anhydrous kuma an mayar da hankali a ƙarƙashin raguwar matsa lamba, don samun fili (a) (175 g, 1 mol, 100%) a matsayin mai ba tare da launi ba.TLC: Rf = 0.5 (50% EtOAc in Hex) wanda aka gani tare da Ce-Mo stain1H NMR (600MHz, CDCl3) delta 1.47 (s, 9H), 2.88 (br s, IH), 3.41 (br s, 2H), 3.76 (br s, 2H).
90.1 g (1.2 mol) na 2- (methylamino) ethanol an narkar da shi a cikin 1.2 L na methylene chloride, 218 g (1 mol) na Boc2O an ƙara shi a hankali a ciki yayin da aka zuga sakamakon da aka samu a 0C, kuma sakamakon da aka samu ya motsa a dakin zafin jiki na 3 hours.An wanke cakudar da aka yi bi da bi tare da 700 ml na cikakken bayani ammonium chloride mai ruwa da 300 ml na ruwa, an bushe shi ta amfani da sodium sulfate mai anhydrous, sannan a mai da hankali a ƙarƙashin matsa lamba don samun 175 g (1 mol) na fili mai achromic wanda aka kiyaye shi. Rukunin Boc (sakamako: 100%).[0140] 1H NMR (600MHz, CDCl3) delta 7.84 (br s, 2H), 7.76 (br s, 2H), 4.34 (d, J = 15.0 Hz, 2H), 3.63 (br s, 2H), 3.04 (d). , J = 15.0 Hz, 3H), 1.46 (d, J = 16.2 Hz, 9H) [0141] 90 g (0.514 mol) na fili da aka samu a cikin 1.5 L na tetrahydrofuran, 88.0 g (539 mol) na N- hydroxyphthalimide da 141 g (0.539 mol) na triphenylphosphine an kara su a ciki, 106 ml (0.539 mol) na diisopropyl azodicarboxylate an kara da shi a hankali yayin da yake motsawa da sakamakon da aka samu a 0C, kuma sakamakon da aka samu ya motsa har tsawon sa'o'i 3 yayin da zafin jiki ya tashi. zuwa zafin jiki.Bayan maida hankali ga cakuda dauki a karkashin rage matsa lamba, 600 ml na isopropylether aka kara a ciki, da sakamakon da aka zuga a 0C na 1 hour, da kuma farin m-type triphenylphosphine oxide aka tace.An wanke daskararre tare da 200 ml na isopropylether sanyaya zuwa 0C kuma an tattara shi tare da filtrate na farko, kuma sakamakon da aka samu ya mayar da hankali a ƙarƙashin rage matsa lamba don samun 198 g na cakuda Compound XX da diisopropyl hydrazodicarboxylate a cikin rabo na 10 zuwa 15%. (sakamako: 120%).[0142] 1H NMR (600MHz, CDCl3) delta 7.84 (br s, 2H), 7.76 (br s, 2H), 4.34 (d, J = 15.0 Hz, 2H), 3.63 (br s, 2H), 3.04 (d). , J = 15.0 Hz, 3H), 1.46 (d, J= 16.2 Hz, 9H)
 Ginin 12, No.309, Titin 2 ta Kudu, yankin raya tattalin arziki, gundumar Longquanyi, Chengdu, Sichuan, kasar Sin.
Ginin 12, No.309, Titin 2 ta Kudu, yankin raya tattalin arziki, gundumar Longquanyi, Chengdu, Sichuan, kasar Sin. amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com
amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com +86 (028) 84841969
+86 (028) 84841969 +86 135 5885 5404
+86 135 5885 5404



















.png)


